Webinar: This panel of leading experts discusses insights from the PRECISE Trial 1-Year data, how the findings relate to the 2021 AHA/ACC Chest Pain Guidelines, and what the data tell us about different strategies for the evaluation and diagnosis of coronary artery disease. Featuring Dr. Pamela S. Douglas, Dr. Maros Ferencik, and Dr. Gregg W. Stone
The Precision Pathway, centered around CCTA+FFRCT, achieved its primary endpoint by significantly reducing the composite of all-cause death, nonfatal MI, or catheterization without obstructive disease relative to Traditional Testing at 1 year.
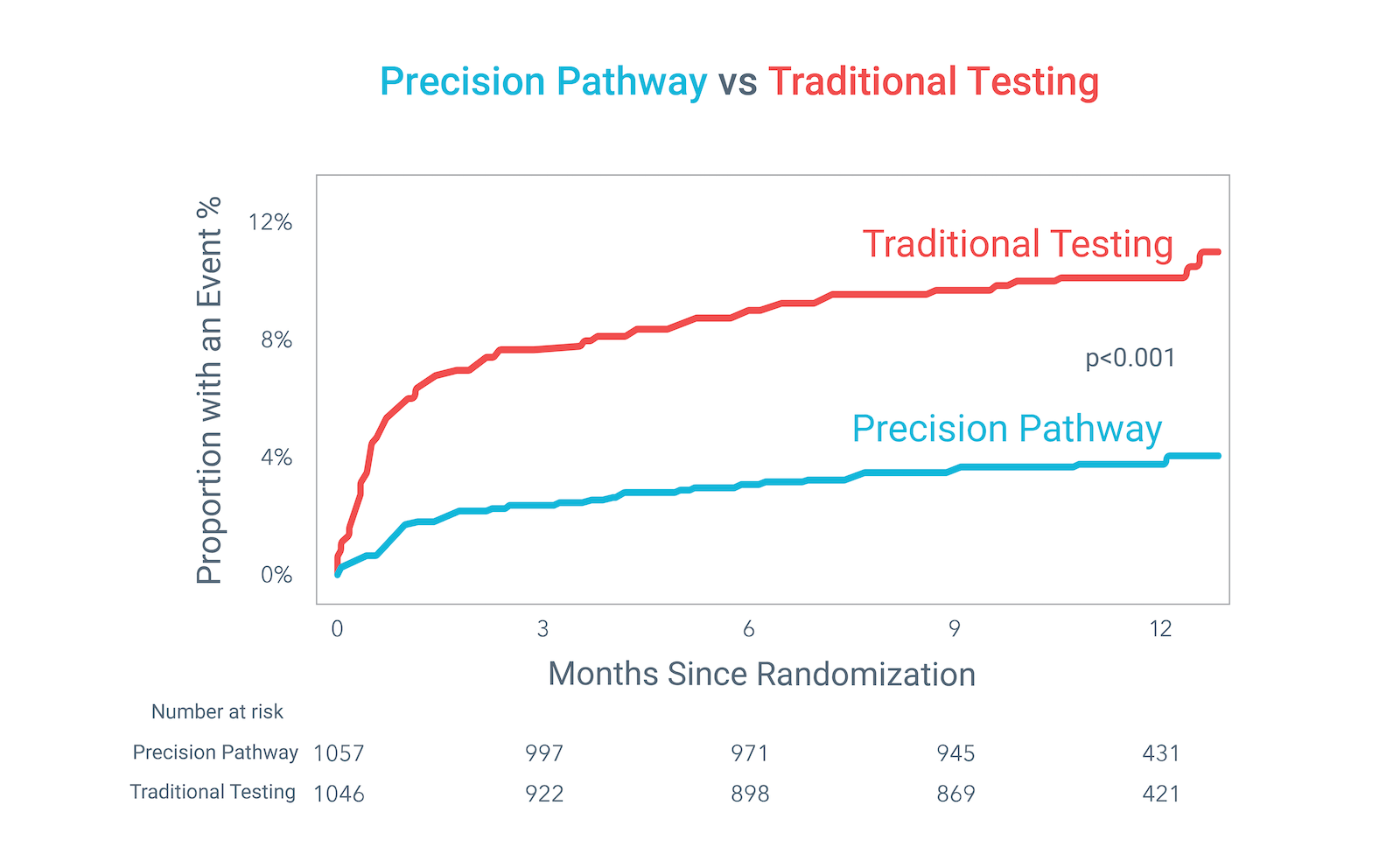
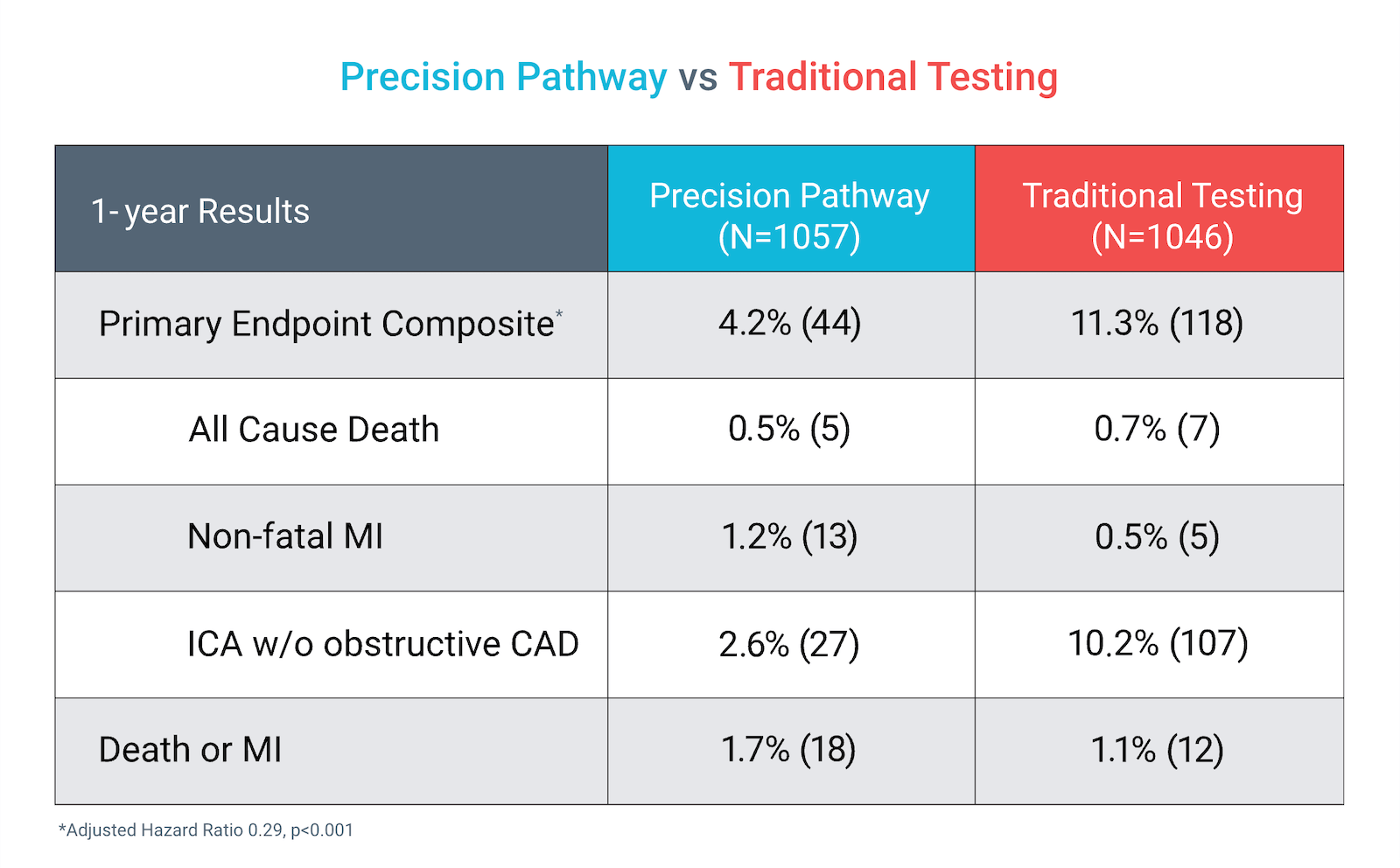


Precision Pathway compared to Traditional Testing:
Global Sites
Patients
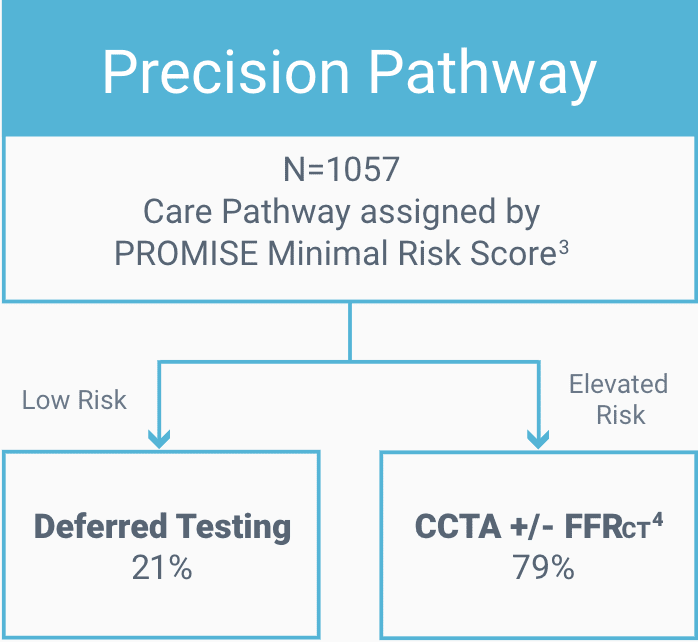
Risk scoring to defer testing for low-risk patients.3
CCTA with selective FFRCT4 for elevated risk patients.
Functional testing (stress nuclear and stress echo) and Invasive Coronary Angiography (ICA).
More accurate non-invasive diagnosis
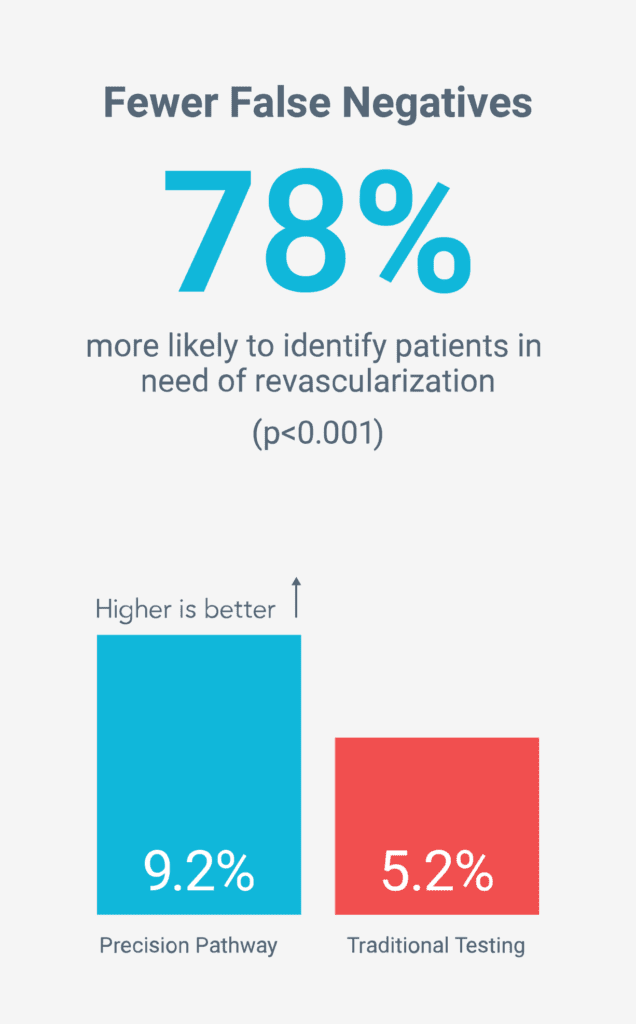
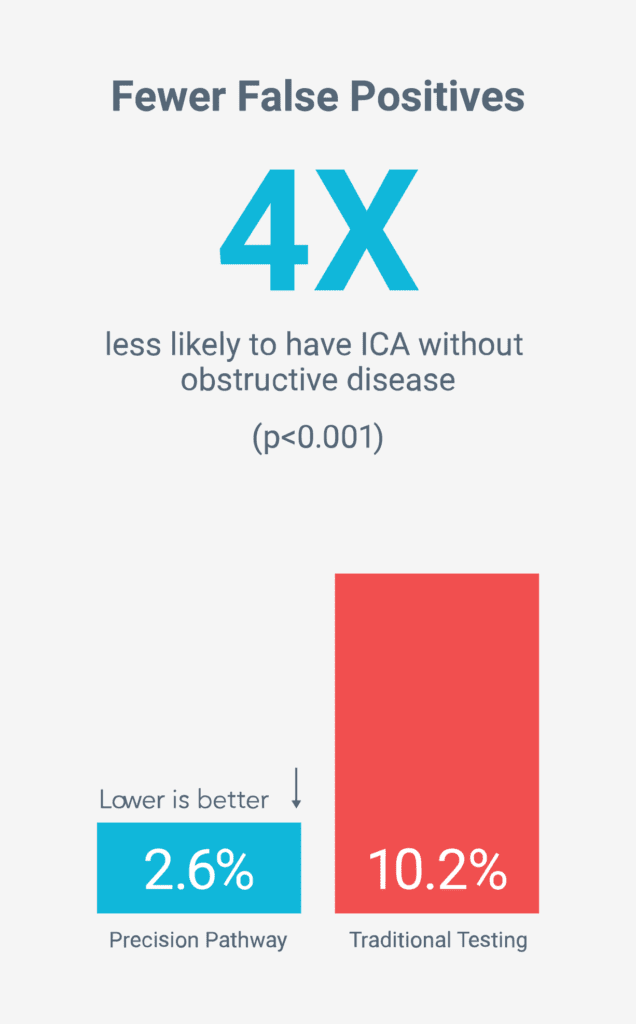
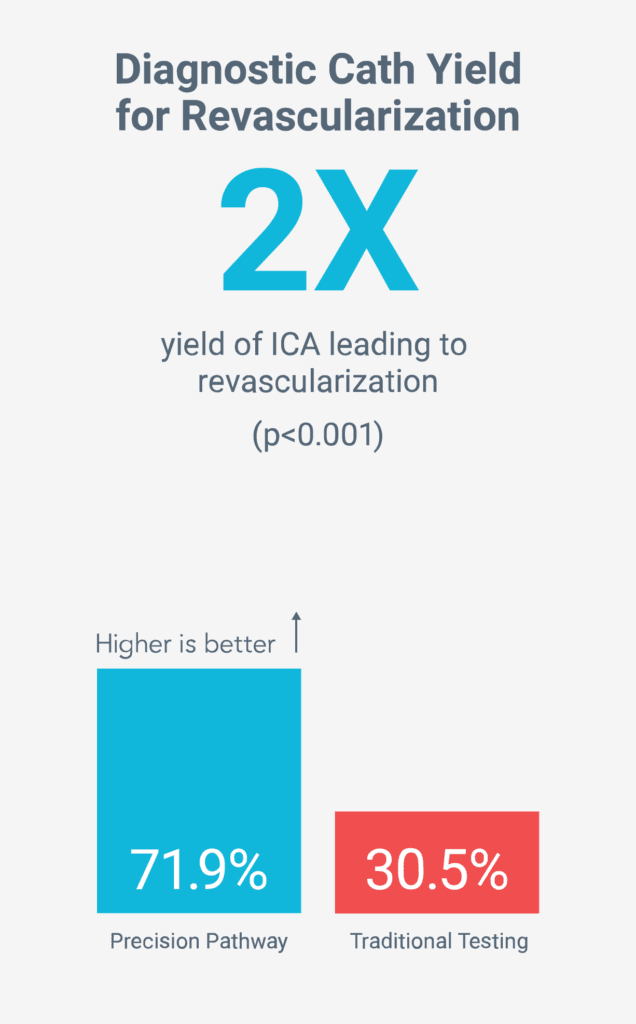
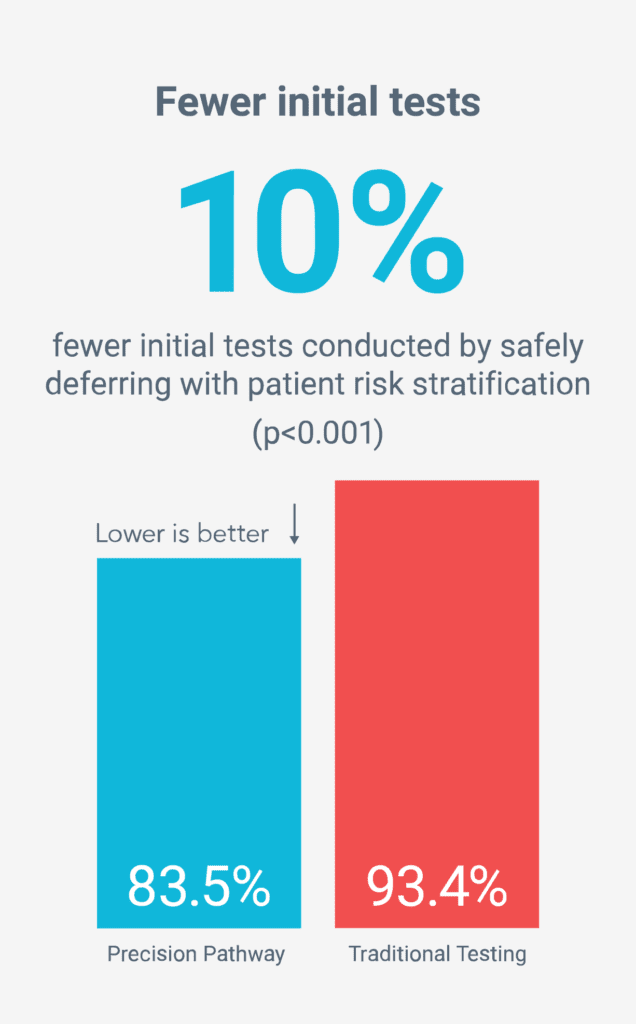
Fewer unnecessary tests
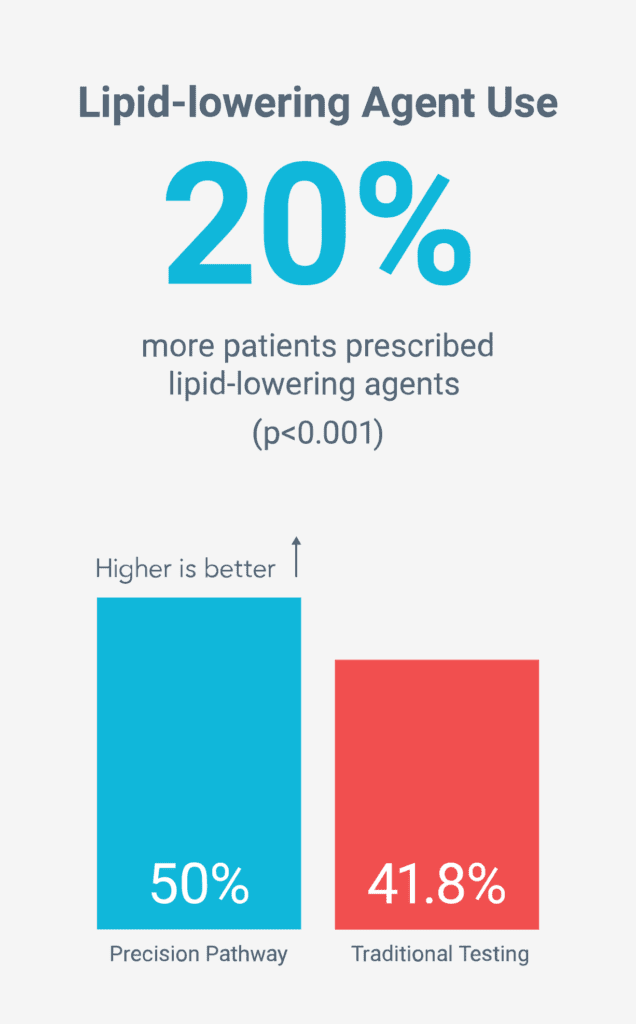
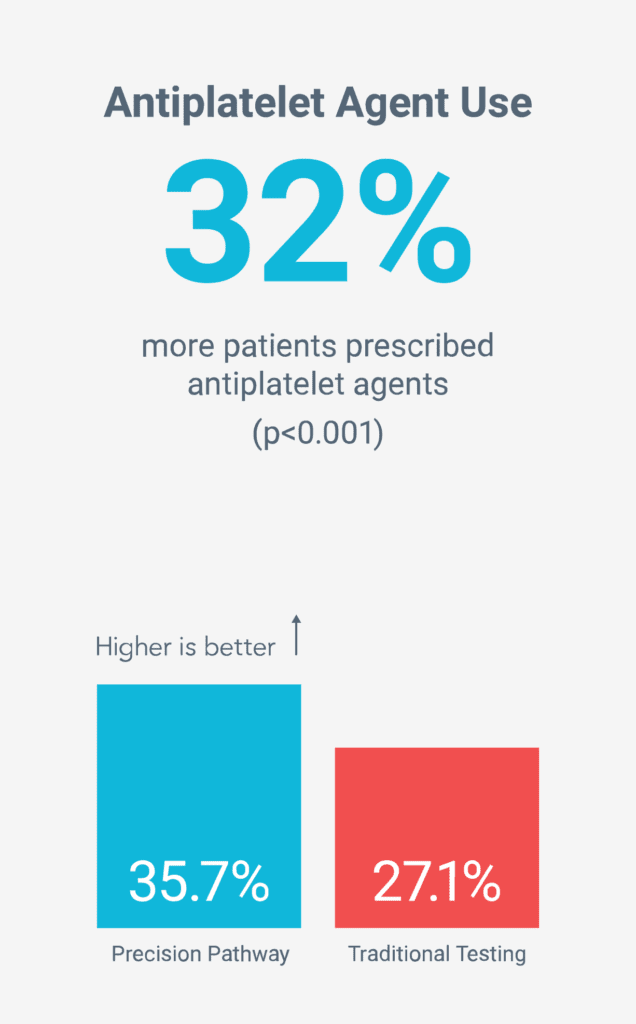
Reduced long-term risk by increasing preventive therapies5
Randomized into one of two pathways.
Randomization stratified by site, PROMISE Minimal Risk Score (Low Risk vs. Elevated Risk), and intended first test (if randomized to Traditional Testing).

All subsequent care and testing decisions made by site clinician.
Guideline-directed medical management recommended for all.
Primary Endpoint (1 year)
Death, Nonfatal MI, Cath without obstructive CAD
Secondary Endpoints
Death, Nonfatal MI, Unplanned CV Hospitalizations, Radiation, Preventive Medication Use, Cath Yield, Resource Use, Quality of Life
REFERENCES




The information provided by the HeartFlow Analysis is intended to be used in conjunction with the patient’s clinical history, symptoms, and other diagnostic tests, as well as the clinician’s professional judgement. The HeartFlow Analysis may not be appropriate for all patients. See indications for use for more information. The HeartFlow Analysis, featuring the FFRCT Analysis, RoadMapTM Analysis, Plaque Analysis, and HeartFlow Planner, has received FDA Clearance in the United States of America. The FFRCT Analysis and HeartFlow Planner are CE Marked in Europe and the United Kingdom and approved in Japan and Canada. The HeartFlow Analysis, featuring FFRCT Analysis, RoadMapTM Analysis, Plaque Analysis, and HeartFlow Planner, is commercially available in the United States. The FFRCT Analysis and HeartFlow Planner are commercially available in the United Kingdom. The FFRCT Analysis is also commercially available in Europe, Japan, and Canada.
© 2024 HeartFlow, Inc. | HeartFlow and the HeartFlow logo are registered trademarks of HeartFlow, Inc. Additionally, RoadMap is claimed as a trademark of HeartFlow, Inc. www.heartflow.com | 331 E Evelyn Ave, Mountain View, CA 94041
*Required fields
If you would like to request to have the HeartFlow Analysis available at a location near you, please submit your information below with details of the institution. We will share this information with the institution, but it will not guarantee HeartFlow will become available.
*Required fields
オンライン提出フォームから研究助成金を申請してください。
HeartFlow FFRCT 分析は、有資格の臨床医による臨床的に安定した症状のある冠状動脈疾患患者への使用を目的とした個別化された心臓検査です。 HeartFlow Analysis によって提供される情報は、資格のある臨床医が患者の病歴、症状、その他の診断検査、および臨床医の専門的判断と組み合わせて使用することを目的としています。
ハートフロー分析に関する追加の適応情報については、次のサイトをご覧ください。www.heartflow.com/indications.
さらに質問がある場合は、このメッセージを閉じてフォームに記入するか、サポート チームにお電話ください。: 877.478.3569.
The HeartFlow FFRCT Analysis is a personalized cardiac test indicated for use in clinically stable symptomatic patients with coronary artery disease by qualified clinicians. The information provided by the HeartFlow Analysis is intended to be used by qualified clinicians in conjunction with the patient’s history, symptoms, and other diagnostic tests, as well as the clinician’s professional judgement.
For additional indication information about the HeartFlow Analysis, please visit www.heartflow.com/indications.
If you have additional questions, close out of this message to complete our form or call our support team: 877.478.3569.Please use our online submission form on the Clinical Research Page to apply for research grants.
Thank you for your interest!

Executive Vice President and Chief Medical Officer
Campbell brings a wealth of experience to HeartFlow, where he serves as the Chief Medical Officer. Prior to joining HeartFlow, he was the Chief Scientific Officer and Global Head of Research and Development at Cordis Corporation, Johnson & Johnson, where he was responsible for leading investments and research in cardiovascular devices. Prior to Cordis, he was Associate Professor of Medicine at Harvard Medical School and the Harvard-M.I.T. Division of Health Sciences and Technology, and Director of the Cardiac Catheterization and Experimental Cardiovascular Interventional Laboratories at Brigham and Women’s Hospital. He served as Principal Investigator for numerous interventional cardiology device, diagnostic, and pharmacology trials, is the author of numerous journal articles, chapters, and books in the area of coronary artery and other cardiovascular diseases, and was the recipient of research grant awards from the NIH and AHA.
He received his A.B. from Harvard College and his M.D. from Harvard Medical School.