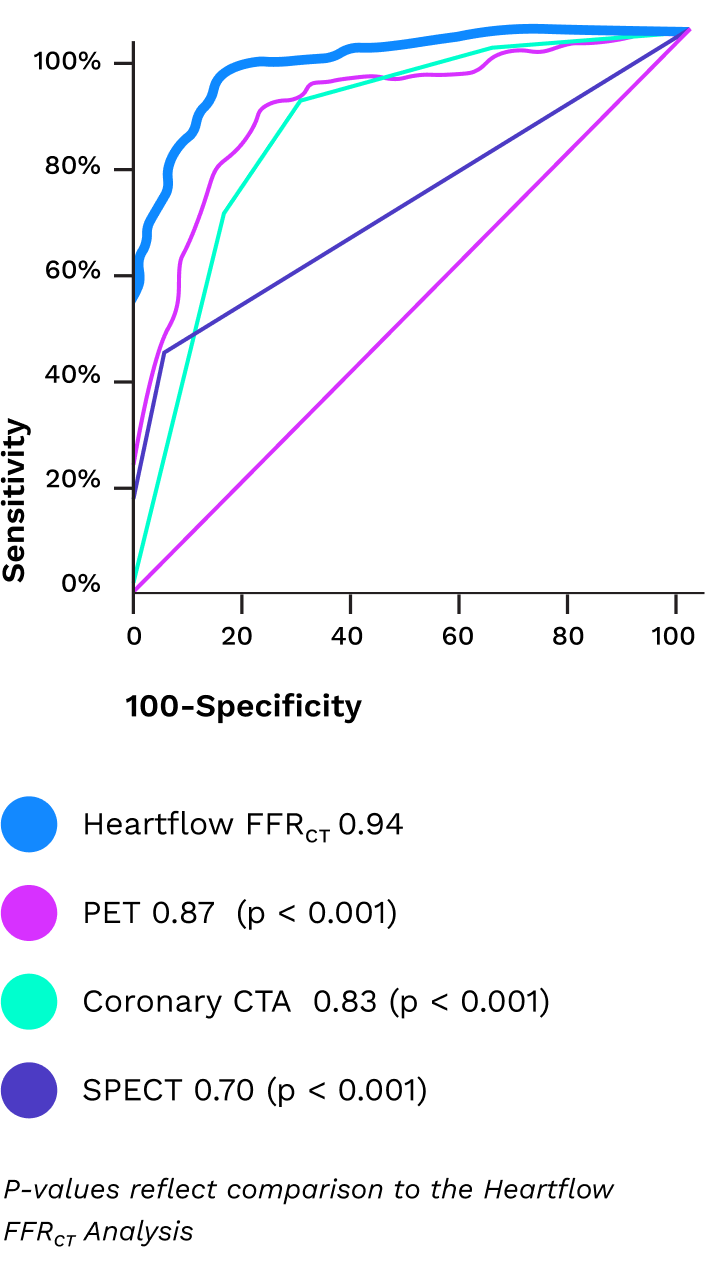
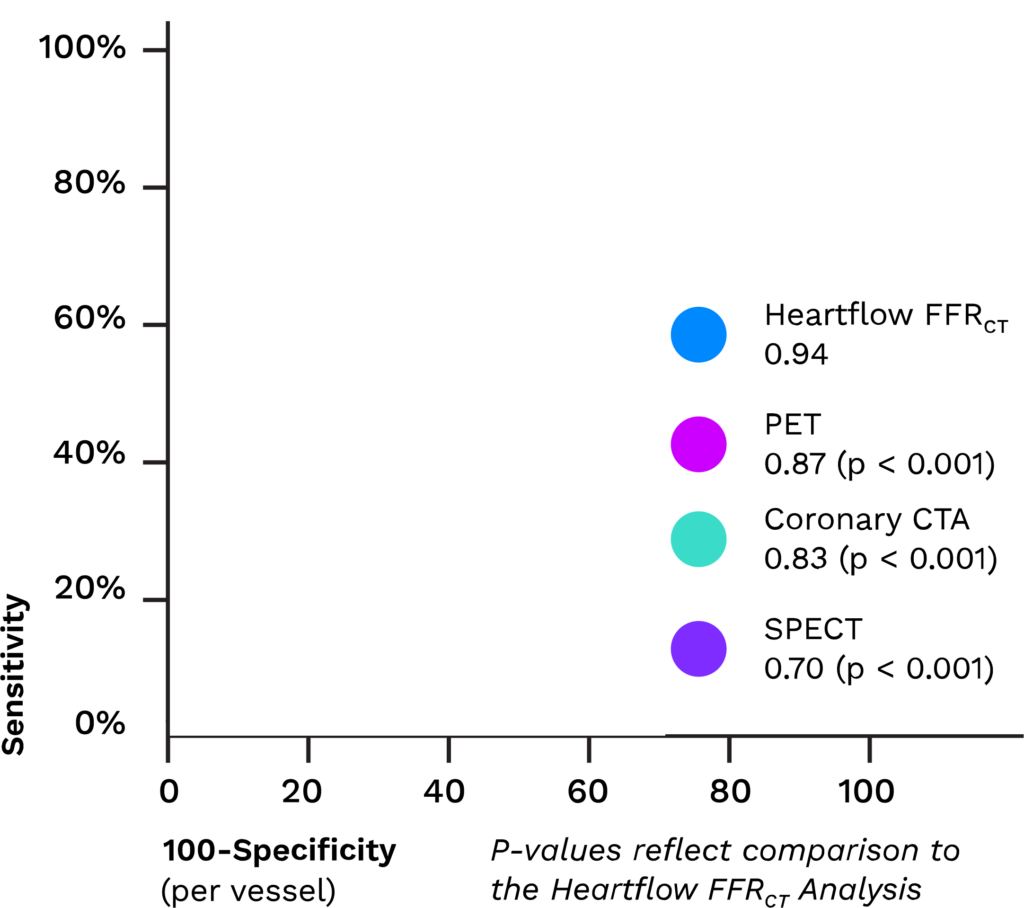
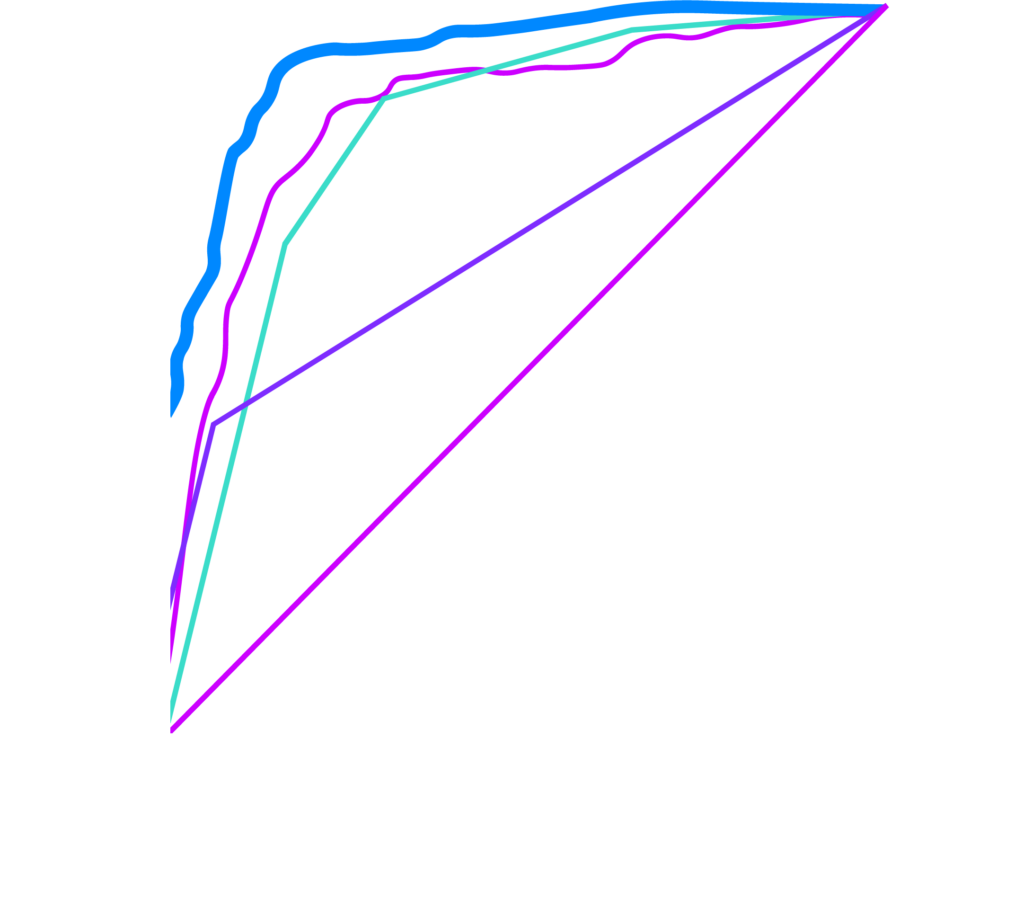
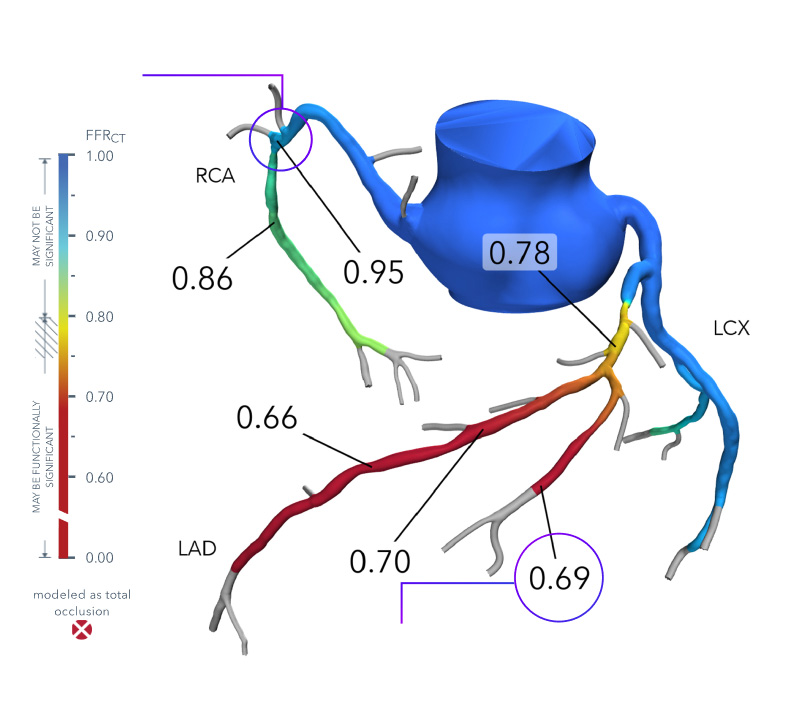
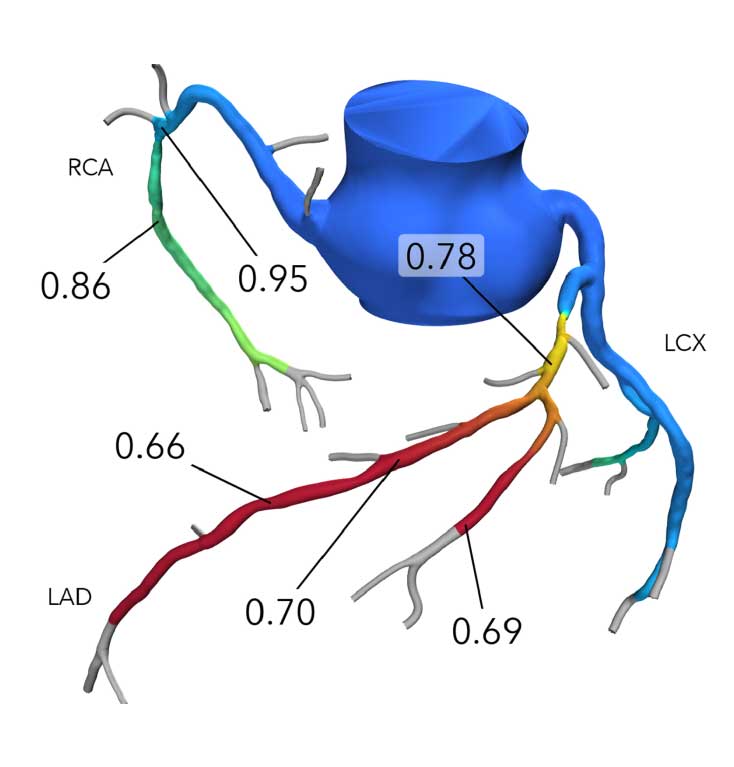
How It Works
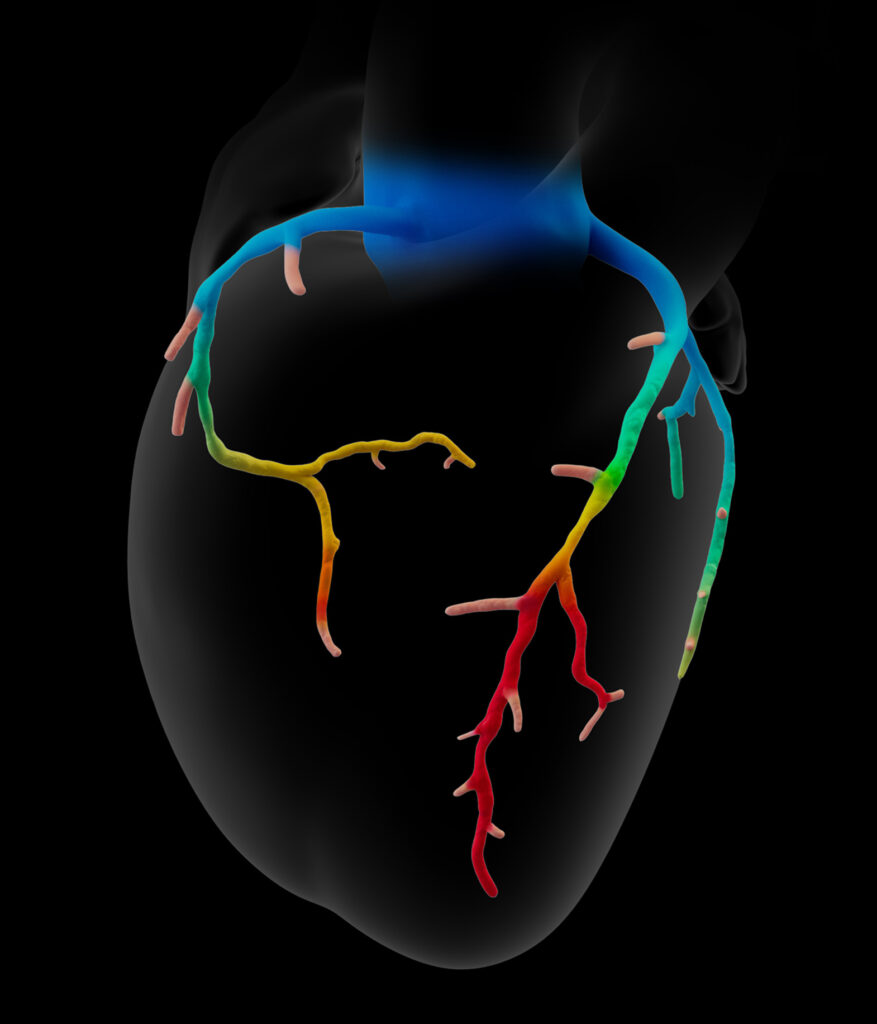
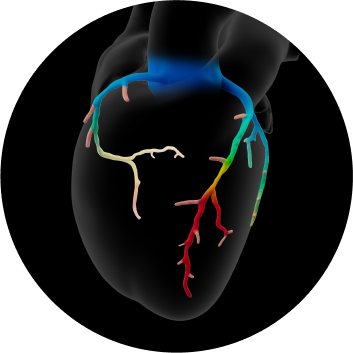
- Patient-specific, interactive, 3D anatomical and physiological reconstruction
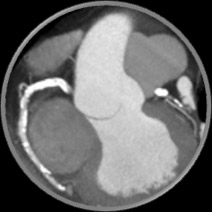
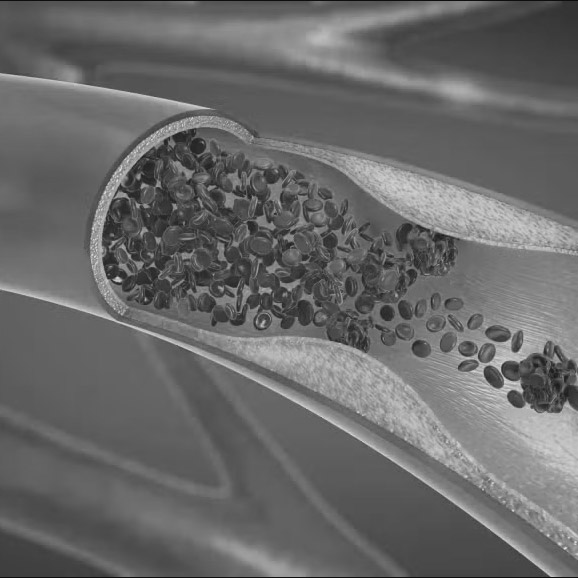
- Rapid assessment of blood flow anywhere in the coronary tree, with color-coding to indicate values
- Evaluate multiple and sequential lesions and determine the lesion with the most significant disease for a targeted interventional approach