Reimbursement
Resources
CCTA Reimbursement Has Been Increased.
CMS has approved an elevated Ambulatory Payment Classification (APC) for Coronary CTA (CCTA), doubling reimbursement in the hospital setting and increasing payments in the Physician Fee Schedule (PFS) setting.
CCTA is the recommended first-line pathway for diagnosing patients with suspected coronary artery disease (CAD). This increase to reimbursement reflects CMS’s understanding of the superior efficiency and effectiveness of the patient pathway. The change in reimbursement more appropriately reflects the value CCTA provides in cardiac care and will help make this technology more widely available.
Hospital Outpatient: OPPS Final Rule
| Product | CY 2024 Final Rule | CY 2025 Final Rule | % Change |
|---|---|---|---|
| CCTA | $175 APC 5571 | $357 APC 5572 | +104% |
| FFRCT APC 5724 | $997 | $1,017 | +2% |
| Plaque APC 1151 | $950 | $950 | — |
Physician Office: PFS Final Rule
| Product | CY 2024 Final Rule | CY 2025 Final Rule | % Change |
|---|---|---|---|
| CCTA | $285 | $318 | +12% |
| FFRCT APC 5724 | $888 | $839 | -5.8% |
| Plaque | Set by MACs | Set by MACs | — |
Ensuring Patient Access — An Established Reimbursement Pathway
The Heartflow FFRCT Analysis is the first non-invasive diagnostic tool that aids clinicians in determining, vessel-by-vessel and lesion-by-lesion, both the extent of an artery’s narrowing and the impact that each narrowing has on blood flow to the heart.
By non-invasively identifying which patients, vessels, and lesions do and do not need intervention, clinicians can optimize appropriate use of invasive testing, reduce healthcare system costs and improve patient quality of life.
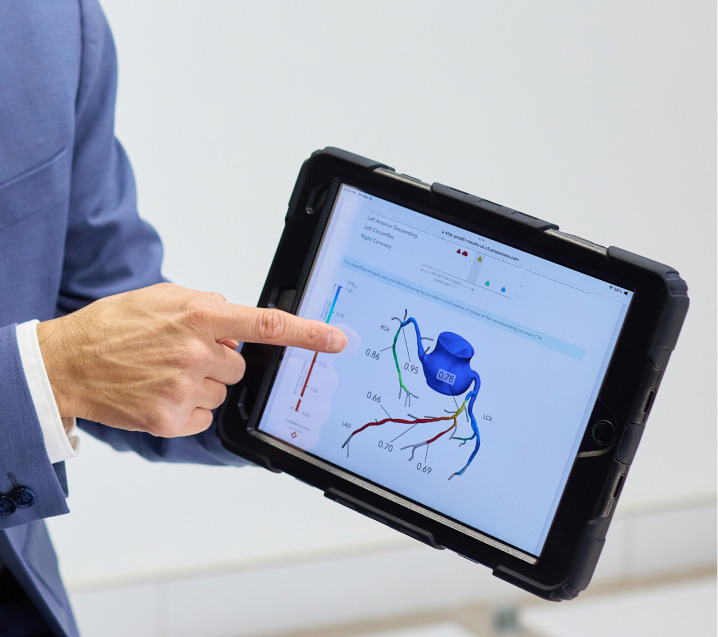
FFRCT Receives
Category I CPT® Code
The American Medical Association (AMA) released new coding guidance for FFRCT Category I code (CPT® 75580). FFRCT must augment physician decision making and requires FFRCT values, interactivity, lesion-by-lesion interrogation, and targeting.
Be confident that Heartflow’s FFRCT Analysis allows you to enable patient care compliantly.
Helping to Achieve the Triple Aim by Improving:
Health of Populations
Reduces unnecessary invasive coronary angiograms (ICA)1Experience of Care
- Helps direct patients to the most appropriate care1
- Provides a better patient experience2